# AEM Technology
# Hydrogen in General
# What is hydrogen?
Hydrogen is the first chemical element of the periodic system. Hydrogen is the most abundant element in the universe. It is the lightest and simplest element we know, one proton and one electron, yet it is high in energy. Hydrogen is an energy carrier and a great multi-talent: it can be transformed into electricity, used as a fuel for transport, used for heating and cooling purposes, as well as various other industrial applications.
# Why do we talk about hydrogen?
We believe that hydrogen will play a central role for the design of modern energy systems to allow for complete green energy independence and security. A burgeoning global industry is taking shape around hydrogen’s potential as a storable fuel or energy carrier. The many advantages it has over battery-electric technology result in hydrogen gaining traction with industry, environmentalists and leading governments. With an abundance of variable renewable energy resources coming on-line, green hydrogen is the solution to power the green energy system of the future.
# Where is hydrogen currently used?
Hydrogen is an energy carrier and as such, a true multi talent. Today, hydrogen is directly used mainly in industrial processes of many kinds, such as ammonia fertilizer production, food processing purposes, the float glass industry, cooling for power plants and in the semiconductor and electronics industry, and many more.
Hydrogen also finds application as a fuel in transport often with water as the only by-product/emission. Vehicles with fuel cells on-board (cars, buses, trains, drones, planes) use hydrogen as the fuel to power their electric propulsion systems. But fuel cells are increasingly important in the power sector also. They can supply power to residential homes, commercial and industrial buildings, and remote locations. They can provide 24/7 power or serve as a backup power device. Hydrogen offers much greater energy storage density and longer autonomy than batteries.
# Why green hydrogen production?
The vast majority, around 99%, of hydrogen used globally is still produced from fossil fuels. Most of that is done by steam methane reforming of natural gas, a process which emits large amounts of greenhouse gases. We speak about green hydrogen when renewable energy sources are used in an electrolyser to make hydrogen from water. Hydrogen is the bridge between renewable power generation and other types of energy vectors and allows us to clean up more than just the electricity sector with fossil-free fuels.
# Why does it make sense to couple hydrogen with intermittent renewable energy sources?
The world has reached a turning point in our understanding of energy. Solar and wind are the two fastest growing energy sources. While governments and industry increasingly understand that fossil fuels are a thing of the past, the challenge remains to make solar and wind useable when we need them. Variable renewables are competitive, and customers are increasingly demanding reliable, secure and independent energy supply from sustainable sources. On-site green hydrogen production allows for complete green energy independence and security. A burgeoning global industry is taking shape around hydrogen’s potential as a storable fuel or energy carrier and many advantages over battery-electric technology result in hydrogen gaining traction with industry, environmentalists and leading governments.
# What are the losses over time through leakage when stored in a tank? Does hydrogen have an “expire date”?
When properly stored, there are no losses. Unlike diesel for example, hydrogen does not have an expiry date and can be stored for years.
# Is hydrogen safe?
Hydrogen is a flammable gas and like with any other gas, appropriate safety measures when handling it must be ensured at all times. Hydrogen’s properties make it safer to handle than commonly used fuels. It is non-toxic, and it is an element lighter than air, so, it will quickly disperse in case of a leak. When planning a hydrogen system installation, it is important to implement appropriate safety measures, such as ventilation and leak detection.
# What is the energy content of hydrogen?
The energy content of hydrogen is described by its (lower and higher) heating value. The lower heating value of hydrogen can be expressed as 33.33 kWh/kg or 3.00 kWh/Nm³. The higher heating value is 39.39 kWh/kg or 3.54 kWh/Nm³. The lower heating value of 3 kWh/Nm³ is usually used if the hydrogen is not burned directly.1 A practical medium value to keep in mind is roughly 3 kWh/Nm³. The energy content of 1 Nm³ (=1000 NL) hydrogen gas is equivalent to 0,36 L gasoline, 1 L liquid hydrogen is equivalent to 0,27 L gasoline and 1 kg hydrogen is equivalent to 3.3 kg gasoline (based on the lower heating value).2
# How much does hydrogen weigh?
The weight of hydrogen is 0.08988 g/L or 0.08988 kg/Nm³.2
# How much hydrogen can be produced by Enapter’s electrolyser and how long does it take to fill a 500 L tank?
Enapter’s electrolysers produce 0.5 Nm³/h (500 NL/h) or 0.04494 kg/h. One electrolyser module produces 12 Nm³ of hydrogen gas in 24 hours, weighting >1 kg (1.0785 kg). At the normal output pressure of the electrolyser with 35 barg, 1.0785 kg of hydrogen occupies a volume of 0.343 m³ (343 L).
A full tank of hydrogen for a passenger vehicle contains about 5 kg of hydrogen gas (stored at 700 barg) and can drive for over 500 km.
Filling up a 500 L tank with one EL running at 100% and at 35 barg takes about 500 L * 35 / 500 NL/h = 35 h until it is full.
The Enapter devices can produce and dry hydrogen 24 hours a day and 7 days per week. They do not need recovery times.
# Enapter's AEM Technology
# What is Enapter's AEM technology, and how does it work?
Enapter's core product is the standardised and stackable anion exchange membrane (AEM) electrolyser. Electrolysers use electricity to split water (H2O) into hydrogen (H2) and oxygen (O2) through an electrochemical reaction. The stack is the electrolyser's heart and comprises multiple cells connected in series in a bipolar design. Enapter's unique technology is the design and operation of these cells, consisting of a membrane electrode assemble (MEA), made from a polymeric AEM and specially designed low-cost electrodes. The anodic half-cell is filled with dilute KOH (alkaline) electrolyte solution; the cathodic half-cell has no liquid and produces hydrogen from water permeating the membrane from the anodic half-cell. Oxygen is evolved from the anodic side and transported out from the stack through the circulating electrolyte. The hydrogen is produced under pressure (typically 35 barg) and already extremely dry and pure (about 99.9%). Using Enapter's ancillary dryer module, hydrogen is delivered at 99.999% purity.
# What is the difference between the Proton Exchange Membrane (PEM) technology and the Anion Exchange Membrane (AEM) technology, and what are the advantages of AEM?
Proton exchange membrane electrolysers (PEM) use a semipermeable membrane made from a solid polymer and designed to conduct protons. While PEM electrolysers provide flexibility, fast response time, and high current density, the widespread commercialisation remains a challenge primarily due to the cost of the materials required to achieve long lifetimes and performance. Specifically, the highly acidic and corrosive operating environment of the PEM electrolyser cells calls for expensive noble metal catalyst materials (iridium, platinum) and large amounts of costly titanium. This poses a challenge to the scalability of PEM electrolysers.
The anion exchange membrane electrolysers use a semipermeable membrane designed to conduct anions. They are a viable alternative to PEM with all the same strengths and several key advantages that lead to lower cost. Due to the less corrosive nature of the environment, steel can be used instead of titanium for the bipolar plates. Furthermore, AEM electrolysers can tolerate a lower degree of water purity, which reduces the input water system's complexity and allows filtered rain and tap water.
# What is the difference between the traditional alkaline and AEM technology, and what are the advantages of AEM?
Traditional liquid alkaline electrolysers have been on the market for quite a while and are relatively cheap. However, they are comparatively slow at responding to a fluctuating power supply, so it is difficult and costly to pair them with renewable energy sources efficiently. Traditional liquid alkaline electrolysers operate with highly concentrated electrolyte solutions and at low pressure. They require additional purification and compression steps to produce high-quality gas at a higher output pressure. This is only cost-effective for centralised and monolithic multi-MW projects.
The AEM electrolyser builds on advantages from traditional alkaline electrolysers, but avoids its weaknesses:
AEM electrolysis works in a highly diluted alkaline environment and is therefore much safer to handle.
The AEM electrolyser can use similarly cost-efficient materials while making much purer hydrogen at higher efficiency.
The AEM electrolyser is fully scalable and is ideal for linking up with variable renewable energy sources.
# Is the electrolyte that Enapter uses for its electrolysers environmental-friendly?
Enapter uses a 1% KOH solution for the electrolysis. It is a clear, soapy liquid which is easily produced by dissolving KOH pallets in purified water. The KOH can be purchased from companies which use green energy for the chemical extraction.
How green the KOH is produced and deposited depends on the manufacturing as well as the disposal management company that you chose. The disposal conditions depend on the company’s health and safety policies as well as the national and local laws and regulations.
However, Enapter electrolysers are not using noble metals which could dissolve in the electrolyte, nor high concentrated chemicals. So, the electrolyte can be disposed of in a more environmental-friendly, easier, and cheaper way compared to other technologies.
# The electrolyser in general
# Where are the electrolysers manufactured? Where is Enapter producing its electrolysers?
Currently, all production takes place in Crespina, Italy, close to Pisa. Enapter is presently preparing a mass production site in Saerbeck, Germany.
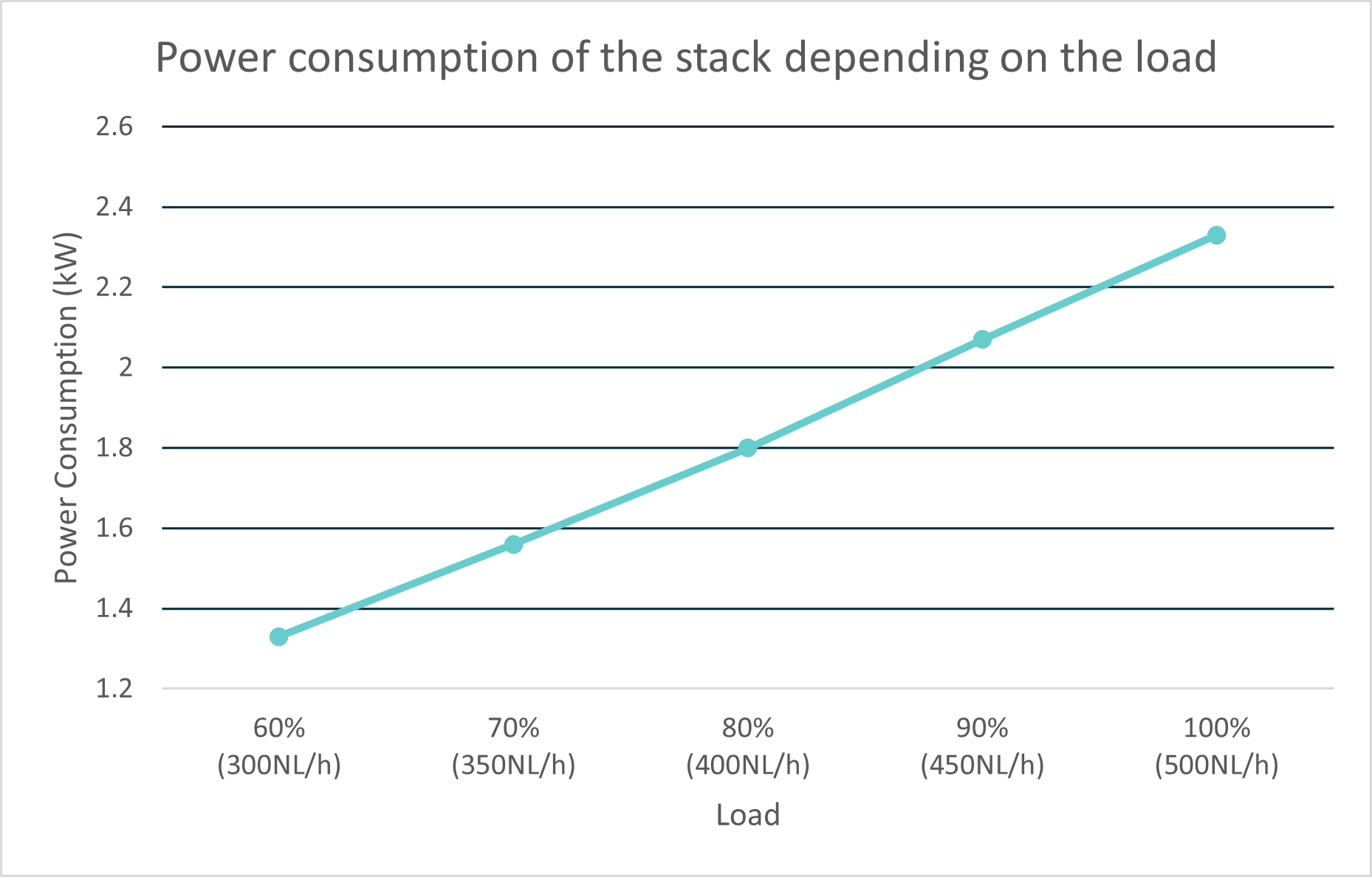
# What is the lowest production rate? How much hydrogen is produced on the lowest production rate and how is the efficiency changing at partial load? How does the polarization curve look like for the stack?
The lowest production rate of the EL 4.1 is 60% of the 500 NL/h, meaning 300 NL/h. The lowest production limit was set to 60% to ensure the devices' safety. The amount of hydrogen in the vent line is then still well below the flammable limits. The energy consumption decreases roughly linearly with the production rate setpoint. The power consumption at a given production rate can be seen in the graph below. Also, the water consumption depends on the hydrogen production.
With the electrolyser control system algorithm, ramping up the production rate by 10% takes about 21sec. Ramping down by 10% takes less than 1sec.
# What is the duration of starting the electrolyser until it is fully functional? How long is the warm-up/ramp-up time?
The ramp up time of the electrolyser depends on the electrolyte temperature (the ramp-up is slower at cooler temperatures and quicker at warm temperatures). In most cases, the system will start with a hydration period of 60 seconds, and then ramp up to the nominal production rate with the following values:
• Warm-up time (time taken for the electrolyser to heat up): The electrolyte working temperature in the electrolyser is 55°C. The electrolyser reaches a heating ratio of about 1 °C/min and reaches maximum efficiency at 55°C. That means, if the machine is started with an electrolyte temperature of 25°C, it will take about 30 min to be fully operational and perform at its maximum efficiency.
• Ramp up time (time to reach nominal production rate): Usually, the 500 NL/h production rate is reached after about 2/3 of the total warm-up time (the warm-up time is 30 min, so if starting at 25°C, it will need 20 min to reach the maximum production rate).
• Build pressure time: When the system starts, and the electrolyser starts to heat up, the hydrogen production begins immediately, and the maximum production rate is reached later. With standard setpoints, the pressure is built up completely in 1/6 of the total warm-up time (if you start at 25 °C, then the warm-up time is 30 min, so 5 min to build up pressure are needed).
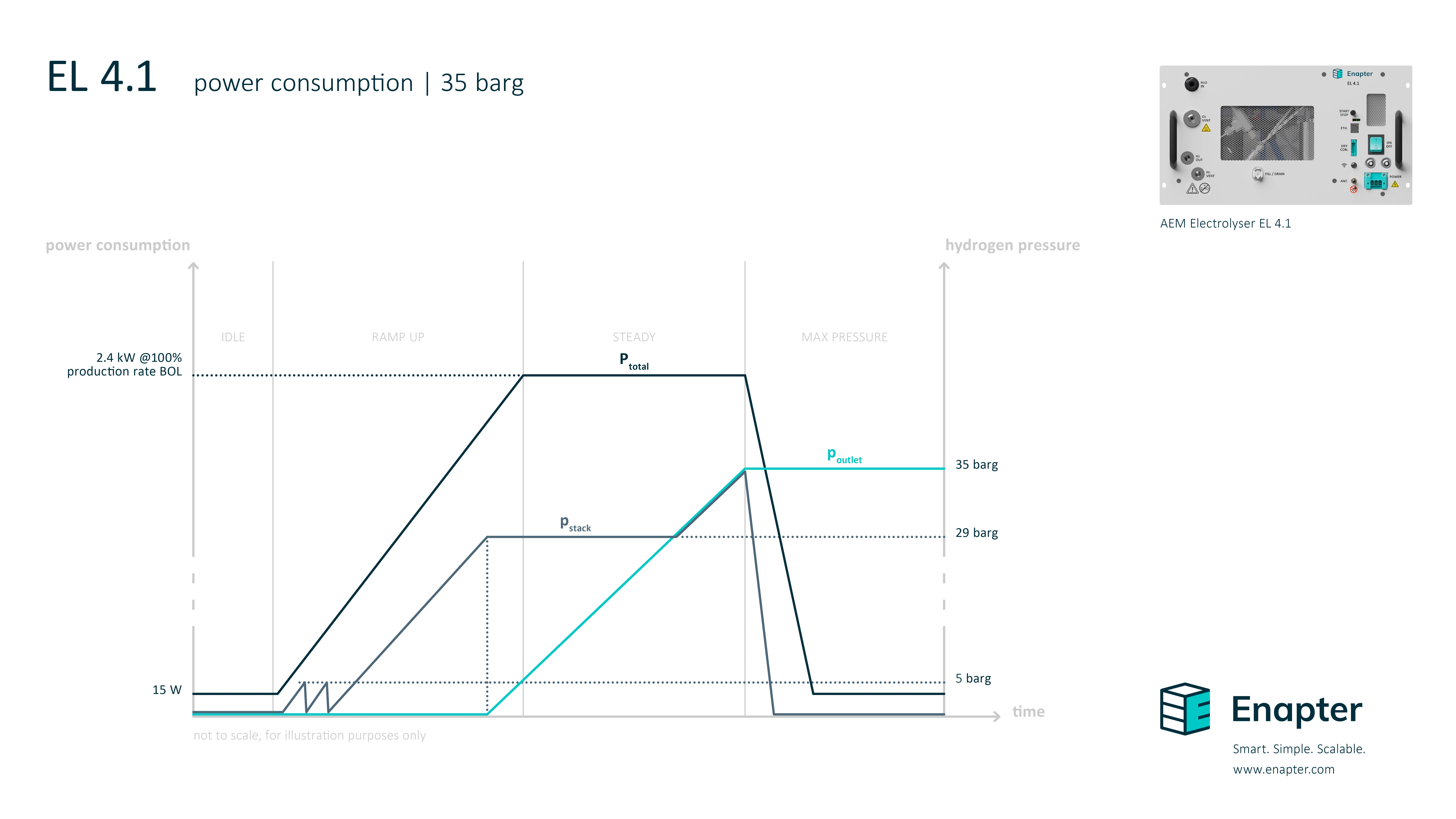
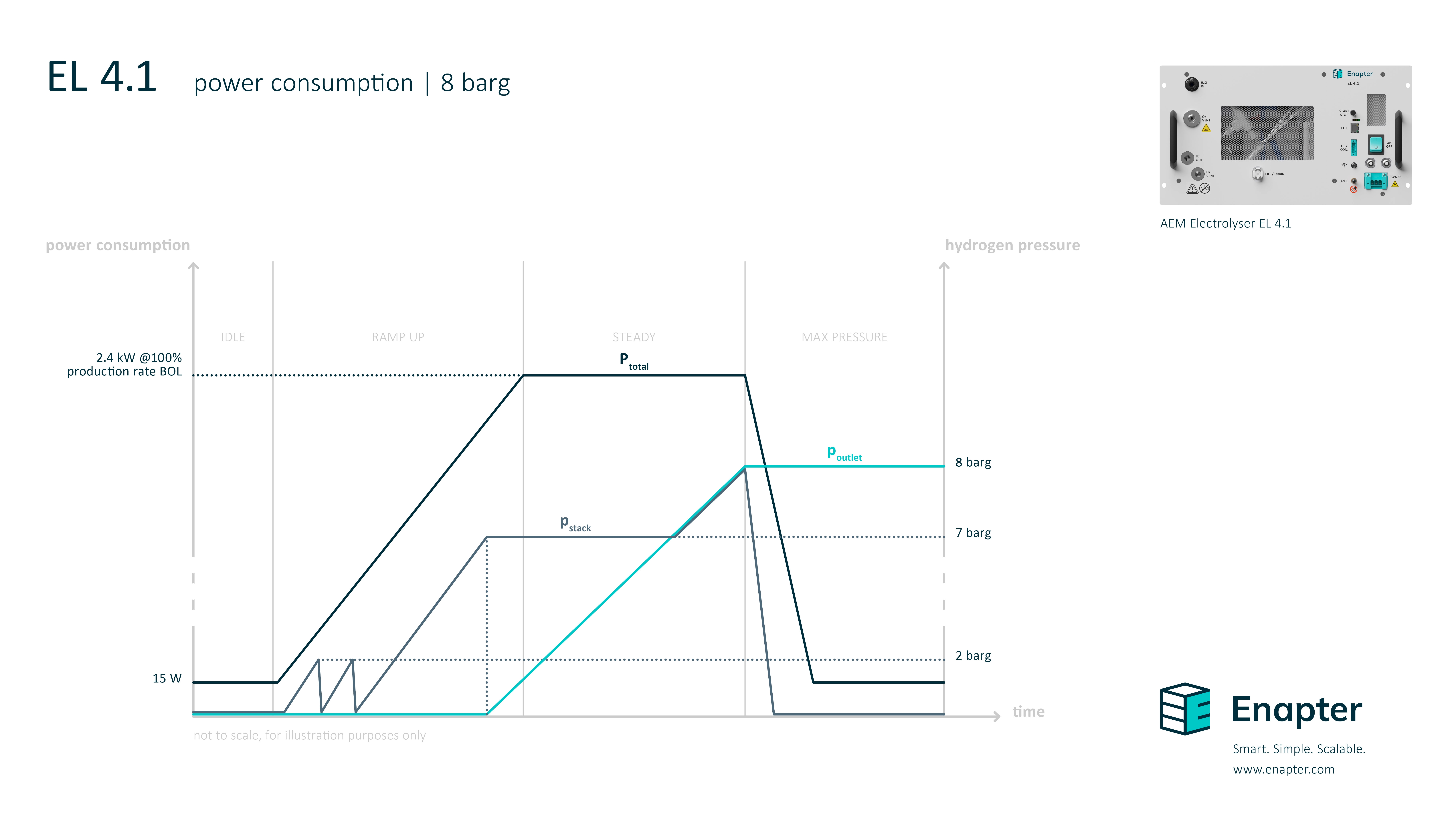
# What’s the power consumption during the start up and what’s the difference between the internal hydrogen pressure and the outlet pressure?
The following diagram shows how the pressure and power consumption increases during start up until it reaches the maximum pressure at the outlet. The values can be adapted to the operator’s preferences.
This is the diagram for the 8 barg version:
# Do frequent start/stop cycles and ramping affect the electrolyser's longevity or performance?
As with all electrochemical devices, our AEM electrolyser stack’s lifetime is shortened with frequent start/stops. With increasing experience in the field and operational data, we can now recommend our customers to limit the electrolyser’s operative cycles to a maximum of five on/off cycles per day, and one on/off cycle per hour. This helps to ensure the longevity of the electrolyser.
The electrolyser works most efficiently and is most durable when operating continuously. However, our modular design and the Enapter Energy Management System are perfectly suited to accommodate for changing renewable energy supply or fluctuating demand. Individual ELs can be ramped from 60-100%, and the combination of many ELs will allow you to achieve any flowrate needed. If hydrogen demand is intermittent during the day, the addition of an appropriately sized buffer tank can minimise on/off cycles of the electrolyser.
# How to shut down the system?
Shutting down the system is rather easy: either manually by pressing/clicking the stop-button or automatically when the maximum pressure setpoint is reached at the outlet. One thing to note is that after every shutdown, the system will release the internal working pressure and purge a small amount of hydrogen gas from the purge line.
# When does it make sense to not shut down the devices?
It is also possible to leave the devices in standby. Especially during colder temperatures this keeps the internal heater running if no hydrogen is produced. Please consider, that the devices are operated and stored according to the temperature ranges stated in the datasheets.
# How is the water in the electrolyser filled up?
The AEM electrolyser has an internal tank of approximately 3.5 litres. To produce hydrogen, clean water must be provided to the electrolyser via a refilling pipe at a pressure between 1 barg and 4 barg. The electrolyser refills about 1.5 litres of water every 3 hours.
# What is the water input quality requirement for the electrolyser?
The electrolyser is highly resilient to water input and can be fed with purified rainwater or tap water. Simple and cheap reverse osmosis processes with resin filters can provide the required water quality. The water input to the electrolyser needs to be desalinated and have a low conductivity. The lower the conductivity, the better. For details, please see the datasheet. It is not possible to use saltwater in the electrolyser.
# What is water specifications for Enapter AEM Electrolysers?
Detailed Water Specifications for Enapter AEM Electrolysers can be found here.
# Do the electrolysers have built-in conductivity sensors? Can they provide measurement values of the absolute water conductivity value?
The electrolyser does not have a built-in conductivity sensor. Therefore, it is the responsibility of the operator to ensure that the water quality reaches the requirements which can be found in the datasheet. However, the Enapter Water Tank Module has a built-in conductivity sensor which shows a warning and stops the water supply to the electrolysers if the conductivity rises.
# What are the differences between the air-cooled and the liquid cooled electrolyser?
The air-cooled and liquid-cooled electrolyser are nearly identical devices. The only difference is in the heat exchanger subassembly, which has the primary function to maintain a stable electrolyte temperature for the electrolyser operation.
Air-cooled
The air-cooled electrolysers use a fan to blow ambient air to keep the electrolyte at the nominal operating temperature of 55°C. Air with a maximum allowed temperature of 45 °C is taken in at the front of the device and blown out hotter at the back. The operator must ensure distances at the front and the back of the device to allow sufficient air flow.
Pros: uses ambient air, therefore easy and fast to set up
Cons: higher requirements on HVAC and installation space in small rooms or containers
Liquid-cooled
The liquid-cooled electrolysers have a liquid-liquid heat exchanger and use a valve to allow/interrupt the cooling liquid flow. The liquid-cooled version of the electrolyser has minimal air flow requirements for safety purposes and to cool the electronics. Therefore the installation space for the air flow can be reduced depending on the room temperature. It has an additional cooling liquid inlet and outlet on the front panel. The operator must supply pressurized cooling liquid at the inlet, and the device will release the cooling liquid at a higher temperature from the outlet. The temperature increase depends on the supplied pressure and flow rate. The waste heat amount can be found in the datasheet.
Pros: more compact setup as air flow requirements is reduced
Pros: reduced requirements on the HVAC system for indoor installations
Cons: requires the installation of a cooling liquid circuit
Using waste heat
In both the air-cooled and liquid-cooled cases, the total waste heat energy from the electrolysis process is the same. This waste heat, while of relatively “low quality”, could potentially be used by integrators in some specific applications to increase overall efficiency of their energy systems. In most cases however, it is just released to the environment.
# Can CO₂ contamination negatively affect the lifetime of the electrolyser?
CO2 contamination in the air is not a problem for the electrolyser, as the system design avoids potential interaction with the surrounding air. However, CO2 in the electrolyte (e.g. by refilling with carbonised water) reduces the pH value and requires a more frequent electrolyte exchange. When maintained regularly (exchanging the electrolyte), this is reversible and does not contribute to explicit degradation of the electrochemical system.
# Is Nitrogen used during the process?
Enapter's electrolysers do not use Nitrogen.
# The Multicore in General
# What is included in the Multicore? What’s the difference between the hydrogen output purity?
The Multicore contains 420 electrolysis stacks. With optional dryers, the purity raises to 99,999%. A water purification system is needed to provide purified water to the Multicore.
# Can the Multicore be operated continuously if the input power is changing?
The production range can be adjusted between 3% and 105% depending on the available energy.
# Are the stack air cooled or liquid cooled?
The stacks in the Multicore are always liquid cooled.
# Stack specifications
# Which membrane is used for the stack?
Enapter only uses its own developed Anion-Exchange-Membrane (AEM).
# What’s the overall weight, dimensions (length, width, hight) an geometry of the stack?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property. But the stack looks very similar like the own visible in our marketing material.
# How many cells are within one stack?
There are 23 cells in each stack.
# Where can I find the pressure and temperature dependant polarisation curve?
Our stack always works at the most efficient pressure and temperature. Therefore, the polarisation curve is only dependent on the production rate. It can be found in the chapter “The electrolyser in general” above.
# What’s the heat transmission coefficient of the stack?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property.
# What’s the structure, material, length, width, height, and geometry of the bipolar plates?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property.
# What’s the electrical resistance and thermal conductivity, the length, width, height, geometry of the flow channels?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property.
# What’s the electrical resistance and thermal conductivity of the gas diffusion layer and the current collector and what are their materials, porosity, thickness, geometry, and diameter?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property.
# What’s the surface, thickness, ion conductivity, water absorption and pressure resistance of the MEA?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property.
# What’s the electrode’s material and the exchange current density of the catalyst layer?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property.
# What is the surface area of the membrane?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property.
# What is the electrolyser cell’s voltage and current range?
Unfortunately, we cannot provide this information as it is part of Enapter's intellectual property.
# How is the pressure controlled for H₂? Is it using a pressure switch and valve?
Enapter utilises a proportional relief valve to pressurise the system and several pressure transmitters to control and monitor stack and outlet pressures. A solenoid valve opens and closes to return the system to a safe state if an error occurs.
# What is the electrolytic cell rated operating temperature in degree Celsius and degree Fahrenheit?
The rated operating temperature is 55 °C / 131 °F.
1: How is (green) hydrogen produced by electrolysis? (DE) (opens new window)
Was this page useful?